INDUSTRY NEWS
Veterinary Infection Control Plans: Don’t Forget Aerosol Disease Transmission Prevention
July 29, 2024

Veterinary Settings: A One Health to Infection Control
Veterinary practices and related environments must have a complete infection control plan. We need to think not just about animal pathogens of concern but also about zoonotic diseases, reverse zoonoses, and ways to prevent hospital-acquired infections. To ensure a safe, effective, well-rounded control plan, we must fully understand the interconnection between humans, animals, and the environment. This interplay contributes to how diseases spread, what diseases pose a threat, what risks are in what situation, and more. Simply put, we need to take a One Health approach to ensure we cover all the bases and always prevent disease risks to all interested parties when feasible.
Chain of Infection
To understand hazards that may afflict a veterinary environment, we need to recognize the chain of infection and the ability of an infectious agent to trigger not only infection but a disease state. Infectious agents require various criteria to permit host invasion, create infection, and cause illness.
Infection spread is considered a chain of infection event that includes:1,2
- The individual pathogen
- The infectious reservoir in nature
- The means of egress from the reservoir
- The means of transmission from host to an individual
- The area where that infectious agent enters an individual
- How susceptible each individual is
One agent may have numerous hosts (reservoirs) and several means of transmission (exits the reservoir and enters the host in more than one manner), and, finally, its ability to cause infection varies from one individual to another based on variations in host susceptibility.1
Recognizing Hazards in Infection Control Plans
Veterinary medicine presents unique challenges when it comes to workplace hazards. These include environmental factors, infectious concerns, and animal factors such as teeth and claws. When developing an infection control plan, it is crucial to be as comprehensive as possible when identifying and addressing these risks. This approach ensures we take every feasible step to minimize the risk of disease transmission, spread, and illness, safeguarding all parties’ safety.
When considering infection control plan development, we want to ensure we address our ability to3,4
- Decrease host susceptibility (while also understanding those that are immunocompromised may need additional measures of protection and are at higher risk of infection)
- Decrease host exposure (e.g., by eliminating pathogens via measures that disinfect the air and surfaces, such as we see with using germicidal ultraviolet radiation (GUV))
- Improve host resistance, e.g., improve resilience and ensure healthy immune systems (e.g., consider annual flu shots for employees, wearing facemasks if sick at work)
Finally, establishing active and passive surveillance practices and maintaining proper documentation remains crucial to ensuring the effectiveness of any infection control plan. Equally important is ensuring that all staff know where the plan is written down and understand and follow it. Finally, all team members need to grasp how diseases are transmitted and take necessary precautions to significantly reduce the risk of disease exposure and transmission.3,5
Typical Infection Control Plans
Typical infection control measures that are commonplace in all plans include:3–5
- Hand hygiene and handwashing stations with proper instruction.
- Personal Protective Equipment (PPE), including gloves, masks, gowns, goggles, and booties as warranted. Respirators may be necessary for some diseases, though typically not utilized in most veterinary settings without special training.
- Proper, sound cleaning and disinfection protocols for all facility areas, including reusable equipment, supplies, clothing, etc.
- Appropriate measures to address the disposal of infectious materials.
- Safety equipment, such as gloves, towels, blankets, e-collars, and muzzles, to handle animals safely.
However, in today’s climate, with the increasing prevalence of newly emerging, often zoonotic diseases and given lessons learned from the COVID-19 pandemic, we must step up our infection control plans. Understanding two key modes of transmission helps demonstrate the need for additional measures and thinking beyond surface disinfection and hand hygiene.
Airborne Transmission
Recognizing that airborne transmission encompasses more than just close proximity exposures and surface contamination with fomite risks (droplet transmission), it also includes particulates capable of traveling distances and lingering in the air for long periods of time (aerosol and droplet nuclei transmission), which is paramount to ensuring all avenues of protection are addressed and explored.
Understanding Droplet vs. Aerosol Disease Transmission
One needs to understand disease transmission before determining whether additional protective measures are necessary for any veterinary setting.
Typically, we consider disease transmission occurring via direct and indirect routes.6
- Inhalation (aerosol and droplet transmission)
- Ingestion (water and foodborne illnesses; fecal-oral route)
- Direct contact, including dermal exposure (e.g., wounds, bites, surgical incisions, catheter sites)
- Sexual (vertical) transmission
- Fomites (mechanical transmission)
- Vectorborne
A final route of transmission to consider would be healthcare-acquired infections (HAI), which could arise due to several of the six modes of transmission.
Aerosol Transmission
To understand aerosol transmission, let’s look at the COVID-19 pandemic. Initially, it was presumed that droplet spread was the primary source of infection. However, despite proper hand hygiene practices, facemasks, and other modes of disease prevention, infections were still occurring at an alarming rate. It wasn’t until researchers recognized that aerosol transmission was happening that social distancing (6 feet and less than 15-minute exposure times) was initially suggested, minimizing infection. Further, the institution of means to not just trap virus particles (air purifiers/HEPA filters) but to irradiate or kill the virus while still airborne using germicidal ultraviolet radiation (GUV) demonstrated further improvement in disease prevention. Thus, researchers inferred that in addition to the virus’s ability to spread through the air, remain infectious, travel distances beyond the immediate surfaces, and cause infection when people spend a certain duration of time around the infected, aerosol transmission played a vital role in the transmission cycle, virulence, and ability of the virus to spread rapidly.7–13
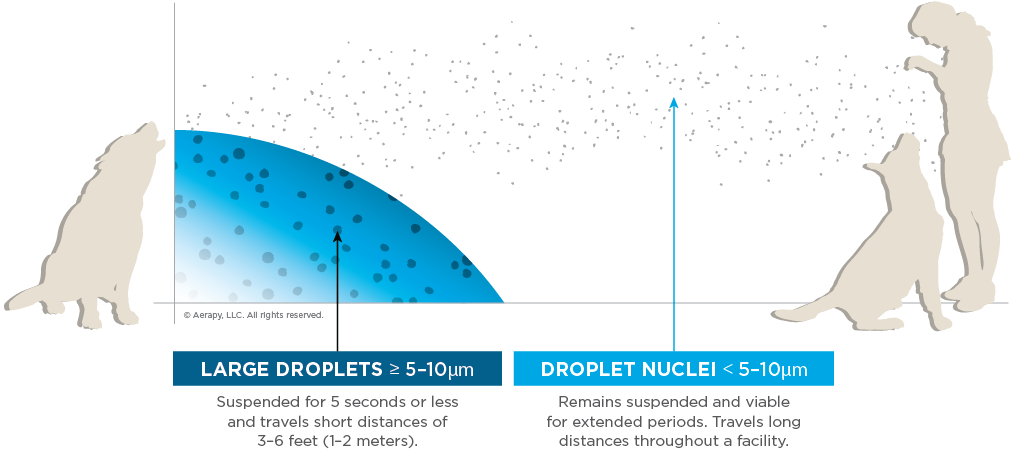
As a result of COVID-19, research evaluating aerosol disease transmission has increased. Consider shelters, grooming facilities, daycares, and vet hospitals where animals congregate. We need to assess the possibility that outbreaks develop or fail to resolve rapidly because of aerosol transmission. When we refer to aerosol transmission, though, what is the distinction? Aerosol transmission involves particles small enough that they do not settle out of the air immediately (< 5 to 10 µm) and can travel distances greater than 3-6 feet (1-2 meters).6,14
Another way to think about aerosol transmission is as droplet nuclei transmission. This refers to particles that are light enough (approximately less than 5 µm) to remain suspended in the air for long periods, traveling significant distances and throughout a facility. This means of transmission can occur not just via straight-line exposure but also through air currents and HVAC ventilation systems. The distance and time these particles travel can vary with humidity and other environmental conditions.
Droplet Transmission
Droplet transmission refers to the spread of infectious particles through various respiratory actions. A contagious agent is released into the air and evaporates into smaller droplet nuclei or remains as larger droplets. Droplet nuclei can travel long distances and remain viable for extended periods, whereas larger droplets typically travel shorter distances, falling to the ground and creating close-contact transmission risks.
While droplets generally travel short distances of only 1-2 meters, are suspended for 5 seconds or less, and are > 5-10 µm in size, constituting close-distance transmission.
When considering the infectious dose of a droplet, we consider it spreading via sneezing, barking, panting, coughing, or any manner in which respiratory secretions are expulsed. With droplet transmission, we focus on the proximity and duration of that close-distance contact (distance from the source and time of exposure).6,15,16
However, these droplets can still become aerosolized if evaporation and other environmental factors permit them to remain suspended as droplet nuclei long enough while remaining infectious. Additionally, we can see larger droplets created via actions taken to benefit the patient, such as bronchoscopy, abscess lancing, suctioning the airway, dentistry, sweeping, high-pressure spraying (should be avoided at all costs), and even routine vacuuming.6 Thus, we want to minimize all forms of aerosolization. If we generate these larger particulates, we want to prevent their spread and eliminate them when feasible.
Variables Affecting Disease Transmission Modalities
Some diseases cause infection by more than one mode of transmission (e.g., droplet, aerosol, and even fomite). When considering respiratory pathogens, we must contemplate various factors about the pathogen and the environment to help determine the risks of aerosol vs. droplet transmission.
Even organisms not historically thought to transmit via aerosol (traveling distances) can do so if the environmental conditions support suspension in the air as droplet nuclei, carry distances, and remain infectious despite normal evaporation processes that occur. However, if an infectious dose can be sustained in the environment, travel distances depend on numerous factors. Key factors include:11,15–22
- Particle size
- The distance a particle is capable of traveling
- Environmental conditions, including relative humidity, temperature, airflow, and ventilation
- The efficiency of the HVAC system
- The ability of a pathogen to travel before settling out of the environment
Additional factors of the pathogen itself, including infectious dose, virulence, and type of infection (lower vs. upper airway), further contribute to its ability to cause disease.
Potential for Aerosol Transmission in Vet Med
While research in veterinary medicine is still ongoing to fully elucidate those diseases truly capable of aerosol transmission, what historically were considered able to spread via droplets only are now likely to be able to spread via aerosol, including as droplet nuclei.
While early research categorized many feline upper respiratory tract infection-causing organisms (URI) and those in the canine infectious respiratory disease complex (CIRDC) as sole droplet transmitted, that doesn’t explain how newer research, e.g., by Jaynes et al., which shows that outbreaks can be stamped out or prevented with the use of multimodal infection control plans that incorporated the use of upper-air GUV among their control modalities.4,18,19,23,24 Thus, implying that the organisms were, in fact, aerosolized. Additional research is ongoing and needed to further quantify the agents that truly have aerosol transmission.
Some diseases, however, it is already apparent through evidence-based research that they are spread via airborne aerosol transmission, including as droplet nuclei, such as M. tuberculosis, Yersinia pestis, and Francisella tularensis.4,6
Support for GUV in Infection Control
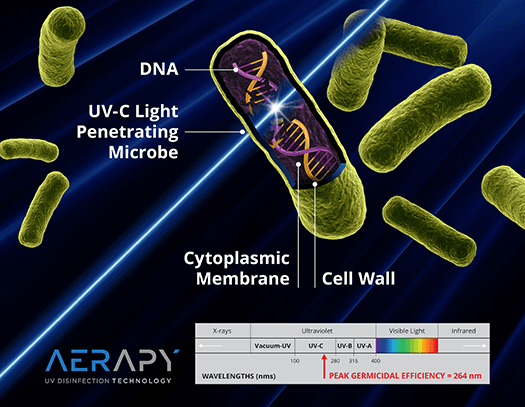
GUV, or ultraviolet germicidal irradiation (UVGI), has become a key component of infection control protocols in human and animal medicine because it provides a means to not just trap infectious agents but truly eliminate them, disinfecting the air and protecting animals, humans, and the environment.
The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), The Centers for Disease Control and Prevention (CDC), and the U.S. Environmental Protection Agency (EPA) provide recommendations on various aspects of infection control within multiple environments. All three support the use, based on scientific research and medical evidence of efficacy in reducing infectious particles and preventing disease transmission via GUV.12,25
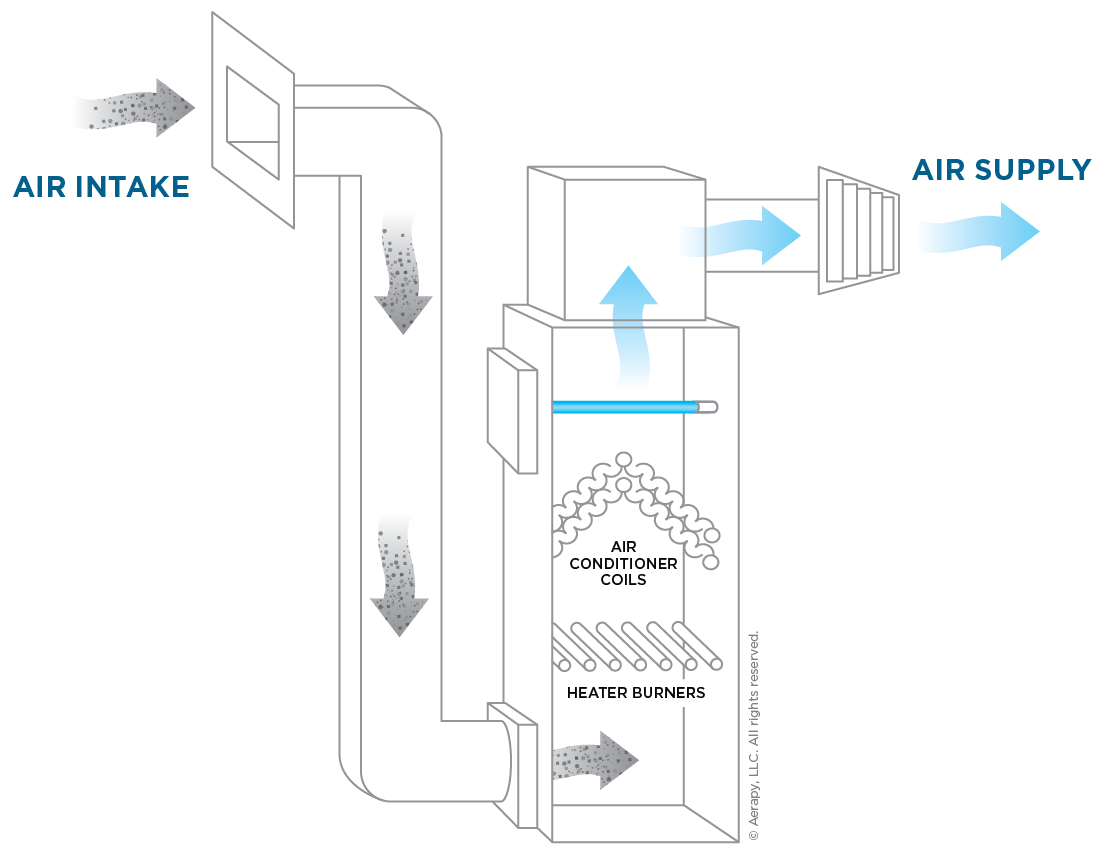
When combined with HVAC systems, air purifiers, and appropriate surface cleaning and disinfection protocols, GUV improves infection control in healthcare settings, including veterinary medicine. This type of air treatment aids disease prevention, decreases biofouling with the air-handling equipment’s cooling coils, and enhances indoor air quality.25
Using UV light via upper-air treatment units, for example, we address those particles and infectious agents that remain on surfaces, people, or near an infectious person or animal. We also address infectious diseases where infectious matter can travel distances and linger in the air (aerosol transmission) for extended periods, spreading to unseen areas of a veterinary hospital’s waiting room, surgery suite, and other regions. It isn’t just for the isolation unit but a tool for overall veterinary protection. It should be part of an all-inclusive infection control plan for every veterinary setting. From boarding facilities to daycares to grooming areas to hospitals and laboratories, using GUV can significantly enhance our ability to protect global health.5,7,8,10,24
To understand GUV and how it may boost your infection control plan to the next level, one must appreciate the distinction between how pathogens are transmitted and factors that impact transmission and recognize the need to implement more than one method to control disease. Viewing infectious disease control through a One Health lens helps us identify key human and animal medicine players that present concerns and hazards for patients, staff, and clients.
A One Health Approach to Infection Control Planning: Incorporating GUV Modalities
When evaluating the hazards associated with a veterinary setting in today’s climate, we must consider the ramifications of poor infection control plans. We must ensure that we evaluate not just those conditions that afflict our patients but also those that can spread from our patients to humans, humans to humans (staff to staff or client and vice versa), and human to animal. We need to ensure we have a comprehensive multimodal infection control plan in place, including one that incorporates not just a means to trap infectious particulates (air purifiers) or disinfect surfaces and individuals but also a means to truly eliminate organisms, such as using GUV. By using this approach, we not only protect the lives of our patients and prevent the spread of the disease but also work in a One Health framework to help preserve the human-animal bond.
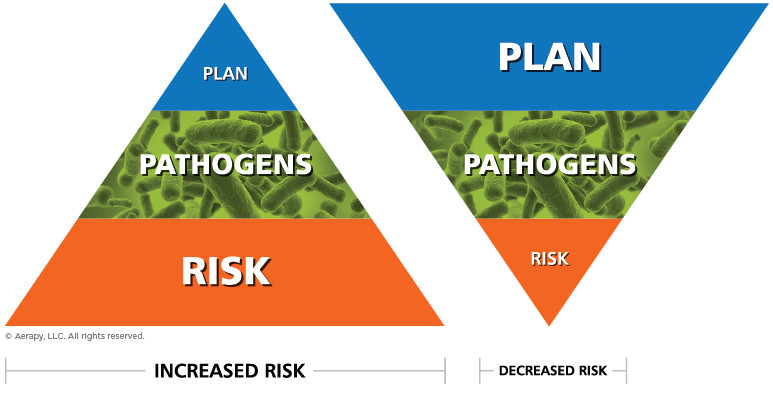
Key Takeaways
References
- Centers for Disease Control and Prevention’s National Institute for Occupational Safety and Health (NIOSH). Chain of Infection Components. The National Institute for Occupational Safety and Health (NIOSH). Published May 22, 2023. Accessed May 24, 2024. (URL)
- The Center for Food Security & Public Health (CFSPH). Iowa State University. Prevention: Aerosol Transmission. Published online 2008. Accessed May 23, 2024. (URL)
- Tattnauer. Infection Control in Vet Clinics – Best Practices. Tuttnauer. Published August 2, 2018. Accessed May 23, 2024. (URL)
- Sykes JE, Weese JS. Infection Control Programs for Dogs and Cats. Canine and Feline Infectious Diseases. Published online 2014:105-118. doi:10.1016/B978-1-4377-0795-3.00011-9
- Morss H. Preventing Transmission of Infectious Disease Among Patients. Today’s Veterinary Practice. 2011; November/December. Accessed May 23, 2024. (URL)
- National Association of State Public Health Veterinarians (NASPHV). National Association of State Public Health Veterinarians Compendium of Veterinary Standard Precautions for Zoonotic Disease Prevention in Veterinary Personnel. Published online 2015. Accessed May 23, 2024. (URL)
- Chiappa F, Frascella B, Vigezzi GP, et al. The efficacy of ultraviolet light-emitting technology against coronaviruses: a systematic review. Journal of Hospital Infection. 2021;114:63-78. doi:10.1016/j.jhin.2021.05.005
- Ragan I, Perez J, Davenport W, Hartson L, Doyle B. UV-C Light Intervention as a Barrier against Airborne Transmission of SARS-CoV-2. Viruses. 2024;16(1):89. doi:10.3390/v16010089
- Fischer RJ, Port JR, Holbrook MG, et al. UV-C Light Completely Blocks Aerosol Transmission of Highly Contagious SARS-CoV-2 Variants WA1 and Delta in Hamsters. Environ Sci Technol. 2022;56(17):12424-12430. doi:10.1021/acs.est.2c02822
- Pearce-Walker JI, Troup DJ, Ives R, et al. Investigation of the effects of an ultraviolet germicidal irradiation system on concentrations of aerosolized surrogates for common veterinary pathogens. American Journal of Veterinary Research. 2020;81(6):506-513. doi:10.2460/ajvr.81.6.506
- Thornton GM, Fleck BA, Fleck N, et al. The impact of heating, ventilation, and air conditioning design features on the transmission of viruses, including the 2019 novel coronavirus: A systematic review of ultraviolet radiation. PLOS ONE. 2022;17(4):e0266487. doi:10.1371/journal.pone.0266487
- Environmental Protection Agency (EPA). What is Upper-Room Ultraviolet Germicidal Irradiation (UVGI)? What is HVAC UVGI? Can either be used to disinfect the air and help protect myself from COVID? EPA. Published March 21, 2022. Accessed May 30, 2024. (URL)
- Beggs PJ. Climate change, aeroallergens, and the aeroexposome. Environ Res Lett. 2021;16(3):035006. doi:10.1088/1748-9326/abda6f
- National Association of State Public Health Veterinarians (NASPHV). A Review of Zoonotic Disease Threats to Pet Owners: A Compendium of Measures to Prevent Zoonotic Diseases Associated with Non-Traditional Pets Such as Rodents and Other Small Mammals, Reptiles, Amphibians, Backyard Poultry, and Other Selected Animals.
- Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infectious Diseases. 2019;19(1):101. doi:10.1186/s12879-019-3707-y
- Wang CC, Prather KA, Sznitman J, et al. Airborne transmission of respiratory viruses. Science. 373(6558):eabd9149. doi:10.1126/science.abd9149
- Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020;8(9):914-924. doi:10.1016/S2213-2600(20)30323-4
- Dear J. Canine Infectious Respiratory Disease Complex. Clinician’s Brief. Published December 2020. Accessed November 1, 2021. (URL)
- Reagan KL, Sykes JE. Canine Infectious Respiratory Disease. Vet Clin North Am Small Anim Pract. 2020;50(2):405-418. doi:10.1016/j.cvsm.2019.10.009
- Cole EC, Cook CE. Characterization of infectious aerosols in health care facilities: An aid to effective engineering controls and preventive strategies. Am J Infect Control. 1998;26(4):453-464. doi:10.1016/S0196-6553(98)70046-X
- Thomas RJ. Particle size and pathogenicity in the respiratory tract. Virulence. 2013;4(8):847-858. doi:10.4161/viru.27172
- Tang JW, Li Y, Eames I, Chan PKS, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-114. doi:10.1016/j.jhin.2006.05.022
- Spiri AM, Meli ML, Riond B, Herbert I, Hosie MJ, Hofmann-Lehmann R. Environmental Contamination and Hygienic Measures After Feline Calicivirus Field Strain Infections of Cats in a Research Facility. Viruses. 2019;11(10):958. doi:10.3390/v11100958
- Jaynes RA, Thompson MC, Kennedy MA. Effect of ultraviolet germicidal irradiation of the air on the incidence of upper respiratory tract infections in kittens in a nursery. Journal of the American Veterinary Medical Association. 2020;257(9):929-932. doi:10.2460/javma.257.9.929
- The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE). Solving Problems: Debunking UVGI Myths. ASHRAE. Published February 2021. Accessed July 11, 2024. (URL)
OVERVIEW
INDUSTRY NEWS
Veterinary Infection Control Plans: Don’t Forget Aerosol Disease Transmission Prevention
July 29, 2024

Veterinary Settings: A One Health to Infection Control
Veterinary practices and related environments must have a complete infection control plan. We need to think not just about animal pathogens of concern but also about zoonotic diseases, reverse zoonoses, and ways to prevent hospital-acquired infections. To ensure a safe, effective, well-rounded control plan, we must fully understand the interconnection between humans, animals, and the environment. This interplay contributes to how diseases spread, what diseases pose a threat, what risks are in what situation, and more. Simply put, we need to take a One Health approach to ensure we cover all the bases and always prevent disease risks to all interested parties when feasible.
Chain of Infection
To understand hazards that may afflict a veterinary environment, we need to recognize the chain of infection and the ability of an infectious agent to trigger not only infection but a disease state. Infectious agents require various criteria to permit host invasion, create infection, and cause illness.
Infection spread is considered a chain of infection event that includes:1,2
- The individual pathogen
- The infectious reservoir in nature
- The means of egress from the reservoir
- The means of transmission from host to an individual
- The area where that infectious agent enters an individual
- How susceptible each individual is
One agent may have numerous hosts (reservoirs) and several means of transmission (exits the reservoir and enters the host in more than one manner), and, finally, its ability to cause infection varies from one individual to another based on variations in host susceptibility.1
Recognizing Hazards in Infection Control Plans
Veterinary medicine presents unique challenges when it comes to workplace hazards. These include environmental factors, infectious concerns, and animal factors such as teeth and claws. When developing an infection control plan, it is crucial to be as comprehensive as possible when identifying and addressing these risks. This approach ensures we take every feasible step to minimize the risk of disease transmission, spread, and illness, safeguarding all parties’ safety.
When considering infection control plan development, we want to ensure we address our ability to3,4
- Decrease host susceptibility (while also understanding those that are immunocompromised may need additional measures of protection and are at higher risk of infection)
- Decrease host exposure (e.g., by eliminating pathogens via measures that disinfect the air and surfaces, such as we see with using germicidal ultraviolet radiation (GUV))
- Improve host resistance, e.g., improve resilience and ensure healthy immune systems (e.g., consider annual flu shots for employees, wearing facemasks if sick at work)
Finally, establishing active and passive surveillance practices and maintaining proper documentation remains crucial to ensuring the effectiveness of any infection control plan. Equally important is ensuring that all staff know where the plan is written down and understand and follow it. Finally, all team members need to grasp how diseases are transmitted and take necessary precautions to significantly reduce the risk of disease exposure and transmission.3,5
Typical Infection Control Plans
Typical infection control measures that are commonplace in all plans include:3–5
- Hand hygiene and handwashing stations with proper instruction.
- Personal Protective Equipment (PPE), including gloves, masks, gowns, goggles, and booties as warranted. Respirators may be necessary for some diseases, though typically not utilized in most veterinary settings without special training.
- Proper, sound cleaning and disinfection protocols for all facility areas, including reusable equipment, supplies, clothing, etc.
- Appropriate measures to address the disposal of infectious materials.
- Safety equipment, such as gloves, towels, blankets, e-collars, and muzzles, to handle animals safely.
However, in today’s climate, with the increasing prevalence of newly emerging, often zoonotic diseases and given lessons learned from the COVID-19 pandemic, we must step up our infection control plans. Understanding two key modes of transmission helps demonstrate the need for additional measures and thinking beyond surface disinfection and hand hygiene.
Airborne Transmission
Recognizing that airborne transmission encompasses more than just close proximity exposures and surface contamination with fomite risks (droplet transmission), it also includes particulates capable of traveling distances and lingering in the air for long periods of time (aerosol and droplet nuclei transmission), which is paramount to ensuring all avenues of protection are addressed and explored.

Understanding Droplet vs. Aerosol Disease Transmission
One needs to understand disease transmission before determining whether additional protective measures are necessary for any veterinary setting.
Typically, we consider disease transmission occurring via direct and indirect routes.6
- Inhalation (aerosol and droplet transmission)
- Ingestion (water and foodborne illnesses; fecal-oral route)
- Direct contact, including dermal exposure (e.g., wounds, bites, surgical incisions, catheter sites)
- Sexual (vertical) transmission
- Fomites (mechanical transmission)
- Vectorborne
A final route of transmission to consider would be healthcare-acquired infections (HAI), which could arise due to several of the six modes of transmission.
Aerosol Transmission
To understand aerosol transmission, let’s look at the COVID-19 pandemic. Initially, it was presumed that droplet spread was the primary source of infection. However, despite proper hand hygiene practices, facemasks, and other modes of disease prevention, infections were still occurring at an alarming rate. It wasn’t until researchers recognized that aerosol transmission was happening that social distancing (6 feet and less than 15-minute exposure times) was initially suggested, minimizing infection. Further, the institution of means to not just trap virus particles (air purifiers/HEPA filters) but to irradiate or kill the virus while still airborne using germicidal ultraviolet radiation (GUV) demonstrated further improvement in disease prevention. Thus, researchers inferred that in addition to the virus’s ability to spread through the air, remain infectious, travel distances beyond the immediate surfaces, and cause infection when people spend a certain duration of time around the infected, aerosol transmission played a vital role in the transmission cycle, virulence, and ability of the virus to spread rapidly.7–13
As a result of COVID-19, research evaluating aerosol disease transmission has increased. Consider shelters, grooming facilities, daycares, and vet hospitals where animals congregate. We need to assess the possibility that outbreaks develop or fail to resolve rapidly because of aerosol transmission. When we refer to aerosol transmission, though, what is the distinction? Aerosol transmission involves particles small enough that they do not settle out of the air immediately (< 5 to 10 µm) and can travel distances greater than 3-6 feet (1-2 meters).6,14
Another way to think about aerosol transmission is as droplet nuclei transmission. This refers to particles that are light enough (approximately less than 5 µm) to remain suspended in the air for long periods, traveling significant distances and throughout a facility. This means of transmission can occur not just via straight-line exposure but also through air currents and HVAC ventilation systems. The distance and time these particles travel can vary with humidity and other environmental conditions.
Droplet Transmission
Droplet transmission refers to the spread of infectious particles through various respiratory actions. A contagious agent is released into the air and evaporates into smaller droplet nuclei or remains as larger droplets. Droplet nuclei can travel long distances and remain viable for extended periods, whereas larger droplets typically travel shorter distances, falling to the ground and creating close-contact transmission risks.
While droplets generally travel short distances of only 1-2 meters, are suspended for 5 seconds or less, and are > 5-10 µm in size, constituting close-distance transmission.
When considering the infectious dose of a droplet, we consider it spreading via sneezing, barking, panting, coughing, or any manner in which respiratory secretions are expulsed. With droplet transmission, we focus on the proximity and duration of that close-distance contact (distance from the source and time of exposure).6,15,16
However, these droplets can still become aerosolized if evaporation and other environmental factors permit them to remain suspended as droplet nuclei long enough while remaining infectious. Additionally, we can see larger droplets created via actions taken to benefit the patient, such as bronchoscopy, abscess lancing, suctioning the airway, dentistry, sweeping, high-pressure spraying (should be avoided at all costs), and even routine vacuuming.6 Thus, we want to minimize all forms of aerosolization. If we generate these larger particulates, we want to prevent their spread and eliminate them when feasible.
Variables Affecting Disease Transmission Modalities
Some diseases cause infection by more than one mode of transmission (e.g., droplet, aerosol, and even fomite). When considering respiratory pathogens, we must contemplate various factors about the pathogen and the environment to help determine the risks of aerosol vs. droplet transmission.
Even organisms not historically thought to transmit via aerosol (traveling distances) can do so if the environmental conditions support suspension in the air as droplet nuclei, carry distances, and remain infectious despite normal evaporation processes that occur. However, if an infectious dose can be sustained in the environment, travel distances depend on numerous factors. Key factors include:11,15–22
- Particle size
- The distance a particle is capable of traveling
- Environmental conditions, including relative humidity, temperature, airflow, and ventilation
- The efficiency of the HVAC system
- The ability of a pathogen to travel before settling out of the environment
Additional factors of the pathogen itself, including infectious dose, virulence, and type of infection (lower vs. upper airway), further contribute to its ability to cause disease.
Potential for Aerosol Transmission in Vet Med
While research in veterinary medicine is still ongoing to fully elucidate those diseases truly capable of aerosol transmission, what historically were considered able to spread via droplets only are now likely to be able to spread via aerosol, including as droplet nuclei.
While early research categorized many feline upper respiratory tract infection-causing organisms (URI) and those in the canine infectious respiratory disease complex (CIRDC) as sole droplet transmitted, that doesn’t explain how newer research, e.g., by Jaynes et al., which shows that outbreaks can be stamped out or prevented with the use of multimodal infection control plans that incorporated the use of upper-air GUV among their control modalities.4,18,19,23,24 Thus, implying that the organisms were, in fact, aerosolized. Additional research is ongoing and needed to further quantify the agents that truly have aerosol transmission.
Some diseases, however, it is already apparent through evidence-based research that they are spread via airborne aerosol transmission, including as droplet nuclei, such as M. tuberculosis, Yersinia pestis, and Francisella tularensis.4,6

Support for GUV in Infection Control
GUV, or ultraviolet germicidal irradiation (UVGI), has become a key component of infection control protocols in human and animal medicine because it provides a means to not just trap infectious agents but truly eliminate them, disinfecting the air and protecting animals, humans, and the environment.
The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), The Centers for Disease Control and Prevention (CDC), and the U.S. Environmental Protection Agency (EPA) provide recommendations on various aspects of infection control within multiple environments. All three support the use, based on scientific research and medical evidence of efficacy in reducing infectious particles and preventing disease transmission via GUV.12,25

When combined with HVAC systems, air purifiers, and appropriate surface cleaning and disinfection protocols, GUV improves infection control in healthcare settings, including veterinary medicine. This type of air treatment aids disease prevention, decreases biofouling with the air-handling equipment’s cooling coils, and enhances indoor air quality.25
Using UV light via upper-air treatment units, for example, we address those particles and infectious agents that remain on surfaces, people, or near an infectious person or animal. We also address infectious diseases where infectious matter can travel distances and linger in the air (aerosol transmission) for extended periods, spreading to unseen areas of a veterinary hospital’s waiting room, surgery suite, and other regions. It isn’t just for the isolation unit but a tool for overall veterinary protection. It should be part of an all-inclusive infection control plan for every veterinary setting. From boarding facilities to daycares to grooming areas to hospitals and laboratories, using GUV can significantly enhance our ability to protect global health.5,7,8,10,24
To understand GUV and how it may boost your infection control plan to the next level, one must appreciate the distinction between how pathogens are transmitted and factors that impact transmission and recognize the need to implement more than one method to control disease. Viewing infectious disease control through a One Health lens helps us identify key human and animal medicine players that present concerns and hazards for patients, staff, and clients.
A One Health Approach to Infection Control Planning: Incorporating GUV Modalities
When evaluating the hazards associated with a veterinary setting in today’s climate, we must consider the ramifications of poor infection control plans. We must ensure that we evaluate not just those conditions that afflict our patients but also those that can spread from our patients to humans, humans to humans (staff to staff or client and vice versa), and human to animal. We need to ensure we have a comprehensive multimodal infection control plan in place, including one that incorporates not just a means to trap infectious particulates (air purifiers) or disinfect surfaces and individuals but also a means to truly eliminate organisms, such as using GUV. By using this approach, we not only protect the lives of our patients and prevent the spread of the disease but also work in a One Health framework to help preserve the human-animal bond.

Key Takeaways
References
- Centers for Disease Control and Prevention’s National Institute for Occupational Safety and Health (NIOSH). Chain of Infection Components. The National Institute for Occupational Safety and Health (NIOSH). Published May 22, 2023. Accessed May 24, 2024. (URL)
- The Center for Food Security & Public Health (CFSPH). Iowa State University. Prevention: Aerosol Transmission. Published online 2008. Accessed May 23, 2024. (URL)
- Tattnauer. Infection Control in Vet Clinics – Best Practices. Tuttnauer. Published August 2, 2018. Accessed May 23, 2024. (URL)
- Sykes JE, Weese JS. Infection Control Programs for Dogs and Cats. Canine and Feline Infectious Diseases. Published online 2014:105-118. doi:10.1016/B978-1-4377-0795-3.00011-9
- Morss H. Preventing Transmission of Infectious Disease Among Patients. Today’s Veterinary Practice. 2011; November/December. Accessed May 23, 2024. (URL)
- National Association of State Public Health Veterinarians (NASPHV). National Association of State Public Health Veterinarians Compendium of Veterinary Standard Precautions for Zoonotic Disease Prevention in Veterinary Personnel. Published online 2015. Accessed May 23, 2024. (URL)
- Chiappa F, Frascella B, Vigezzi GP, et al. The efficacy of ultraviolet light-emitting technology against coronaviruses: a systematic review. Journal of Hospital Infection. 2021;114:63-78. doi:10.1016/j.jhin.2021.05.005
- Ragan I, Perez J, Davenport W, Hartson L, Doyle B. UV-C Light Intervention as a Barrier against Airborne Transmission of SARS-CoV-2. Viruses. 2024;16(1):89. doi:10.3390/v16010089
- Fischer RJ, Port JR, Holbrook MG, et al. UV-C Light Completely Blocks Aerosol Transmission of Highly Contagious SARS-CoV-2 Variants WA1 and Delta in Hamsters. Environ Sci Technol. 2022;56(17):12424-12430. doi:10.1021/acs.est.2c02822
- Pearce-Walker JI, Troup DJ, Ives R, et al. Investigation of the effects of an ultraviolet germicidal irradiation system on concentrations of aerosolized surrogates for common veterinary pathogens. American Journal of Veterinary Research. 2020;81(6):506-513. doi:10.2460/ajvr.81.6.506
- Thornton GM, Fleck BA, Fleck N, et al. The impact of heating, ventilation, and air conditioning design features on the transmission of viruses, including the 2019 novel coronavirus: A systematic review of ultraviolet radiation. PLOS ONE. 2022;17(4):e0266487. doi:10.1371/journal.pone.0266487
- Environmental Protection Agency (EPA). What is Upper-Room Ultraviolet Germicidal Irradiation (UVGI)? What is HVAC UVGI? Can either be used to disinfect the air and help protect myself from COVID? EPA. Published March 21, 2022. Accessed May 30, 2024. (URL)
- Beggs PJ. Climate change, aeroallergens, and the aeroexposome. Environ Res Lett. 2021;16(3):035006. doi:10.1088/1748-9326/abda6f
- National Association of State Public Health Veterinarians (NASPHV). A Review of Zoonotic Disease Threats to Pet Owners: A Compendium of Measures to Prevent Zoonotic Diseases Associated with Non-Traditional Pets Such as Rodents and Other Small Mammals, Reptiles, Amphibians, Backyard Poultry, and Other Selected Animals.
- Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infectious Diseases. 2019;19(1):101. doi:10.1186/s12879-019-3707-y
- Wang CC, Prather KA, Sznitman J, et al. Airborne transmission of respiratory viruses. Science. 373(6558):eabd9149. doi:10.1126/science.abd9149
- Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020;8(9):914-924. doi:10.1016/S2213-2600(20)30323-4
- Dear J. Canine Infectious Respiratory Disease Complex. Clinician’s Brief. Published December 2020. Accessed November 1, 2021. (URL)
- Reagan KL, Sykes JE. Canine Infectious Respiratory Disease. Vet Clin North Am Small Anim Pract. 2020;50(2):405-418. doi:10.1016/j.cvsm.2019.10.009
- Cole EC, Cook CE. Characterization of infectious aerosols in health care facilities: An aid to effective engineering controls and preventive strategies. Am J Infect Control. 1998;26(4):453-464. doi:10.1016/S0196-6553(98)70046-X
- Thomas RJ. Particle size and pathogenicity in the respiratory tract. Virulence. 2013;4(8):847-858. doi:10.4161/viru.27172
- Tang JW, Li Y, Eames I, Chan PKS, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-114. doi:10.1016/j.jhin.2006.05.022
- Spiri AM, Meli ML, Riond B, Herbert I, Hosie MJ, Hofmann-Lehmann R. Environmental Contamination and Hygienic Measures After Feline Calicivirus Field Strain Infections of Cats in a Research Facility. Viruses. 2019;11(10):958. doi:10.3390/v11100958
- Jaynes RA, Thompson MC, Kennedy MA. Effect of ultraviolet germicidal irradiation of the air on the incidence of upper respiratory tract infections in kittens in a nursery. Journal of the American Veterinary Medical Association. 2020;257(9):929-932. doi:10.2460/javma.257.9.929
- The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE). Solving Problems: Debunking UVGI Myths. ASHRAE. Published February 2021. Accessed July 11, 2024. (URL)